
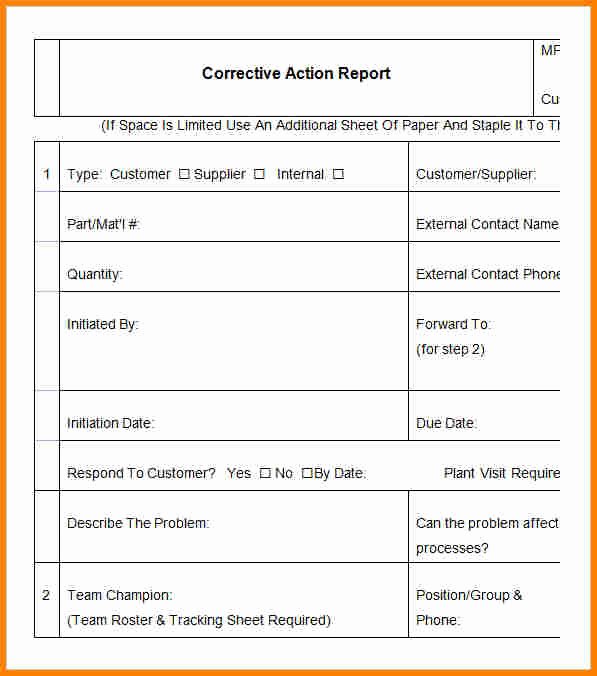
The actions may be in the form of a phone call or an office visit with a qualified research team member. If you become aware of a deviation or unexpected event that endangers the rights, welfare, or safety of participants and others, you must first take immediate corrective actions without first obtaining IRB approval. Step 1: Take Immediate Corrective Actions Research teams must identify, evaluate, and respond to these deviations and unexpected events to protect the rights, safety, and welfare of participants and others and the integrity of the research data. While conducting research, even the most experienced and diligent research teams may deviate from the approved protocol or experience unexpected events.

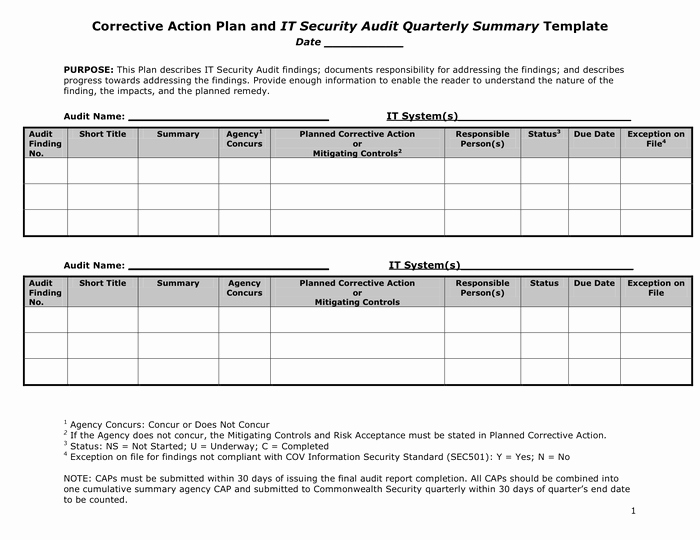
Northwestern Affiliates & Chicagoland PartnersĬorrective and Preventive Action (CAPA) Plans.Northwestern Relying on an External IRB.Northwestern Serving as the IRB of Record.Training Options for External Collaborators.


 0 kommentar(er)
0 kommentar(er)
